Iron-mediated intermolecular N-group transfer chemistry with olefinic substrates†
Abstract
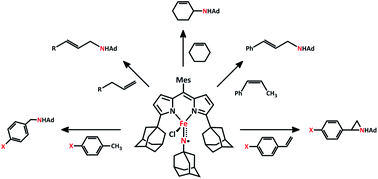
The dipyrrinato iron catalyst reacts with organic azides to generate a reactive, high-spin imido radical intermediate, distinct from nitrenoid or imido species commonly observed with low-spin transition metal complexes. The unique electronic structure of the putative group-transfer intermediate dictates the chemoselectivity for intermolecular nitrene transfer. The mechanism of nitrene group transfer was probed via amination and aziridination of para-substituted toluene and styrene substrates, respectively. The Hammett analysis of both catalytic amination and aziridination reactions indicate the rate of nitrene transfer is enhanced with functional groups capable of delocalizing spin. Intermolecular amination reactions with olefinic substrates bearing allylic C–H bonds give rise to exclusive allylic amination with no apparent aziridination products. Amination of substrates containing terminal olefins give rise exclusively to allylic C–H bond abstraction, C–N recombination occurring at the terminal C with transposition of the double bond. A similar reaction is observed with cis-β-methylstyrene where exclusive amination of the allylic position is observed with isomerization of the olefin to the trans-configuration. The high levels of chemoselectivity are attributed to the high-spin electronic configuration of the reactive imido radical intermediate, while the steric demands of the ligand enforce regioselective amination at the terminal position of linear α-olefins.

 Please wait while we load your content...
Please wait while we load your content...