Reversibly switchable polymer with cationic/zwitterionic/anionic behavior through synergistic protonation and deprotonation†
Abstract
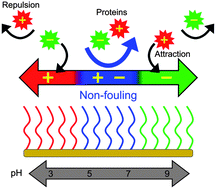
A polymer capable of fully and reversibly switching throughout the entire charge regime is highly desirable for many applications such as drug and gene delivery, controlled ion and molecular transport and tunable filtration membranes. It is essential that for biologically relevant applications the polymer needs to be nonfouling. However, conventional nonfouling zwitterionic polymers have a permanently positive quaternary nitrogen center, making it impossible to switch between charges. Here, we present a rationally designed polymer with a tertiary amine and a carboxylic acid, which is capable of reversibly switching among three distinct charged states, viz., cationic, zwitterionic and anionic, and importantly maintaining the zwitterionic state under physiological pH conditions. Oppositely charged proteins adsorbed on a charged surface selectively can be completely removed by switching the surface to the zwitterionic state. We have also found that these two moieties (i.e., a tertiary amine and a carboxylate moiety) stimulate each other synergistically to achieve a strongly zwitterionic state under physiological conditions and to resist non-specific protein adsorption from undiluted blood plasma and serum when they are close to each other.

 Please wait while we load your content...
Please wait while we load your content...