Novel benzimidazole–oxadiazole hybrid molecules as promising antimicrobial agents†
Abstract
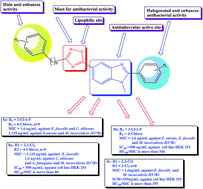
In the present study, we describe the design and expeditious synthesis of novel 2-aryl-5-(3-aryl-[1,2,4]-oxadiazol-5-yl)-1-methyl-1H-benzo[d]imidazole hybrid molecules as promising antimicrobial agents. The core moiety 2-aryl-ethyl-1H-benzo[d]imidazole-5-carboxylate was efficiently synthesized by a rapid ‘one pot’ nitro reductive cyclization reaction using sodium dithionite as reagent. All the compounds were screened for their antimicrobial activities and the active compounds were screened for their anti-tubercular activity against Mycobacterium tuberculosis H37Rv strain by the Microplate Alamar Blue Assay method. Compounds 8k, 8n, 8p and 8r exhibited potent anti-tubercular activity with MIC of 1.6 μg mL−1, which is two times more potent than the standard drugs pyrazinamide and ciprofloxacin and fourfold superior to streptomycin and isoniazid. Further, potent compounds were tested for their preliminary toxicity by hemolytic assay, where the compounds remain nontoxic even at higher concentration and showed good selectivity index. The investigation of cytotoxicity against normal embryonic kidney cell line HEK 293 by MTT assay showed IC50 value of more than 355 μg mL−1 for all the tested potent compounds. From the screening study, 8k, 8n, 8p and 8r emerged as strong candidates with potent antimicrobial activities and good ADME parameters. Attempts were also made to establish the structural activity relationships among the tested compounds.

 Please wait while we load your content...
Please wait while we load your content...