Iodine-mediated synthesis of indazolo-quinazolinones via a multi-component reaction†
Abstract
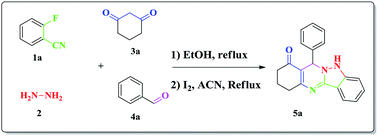
We report an expeditious synthesis of selected indazolo-quninazolinone derivatives via an iodine-mediated multi-component reaction (MCR). The MCR involved an in situ generation of the 1H-indazol-3-amine derivative in ethanol followed by its reaction with the diketone and aryl aldehyde in acetonitrile. A number of compounds have been synthesized using this methodology in good yields.

 Please wait while we load your content...
Please wait while we load your content...