A Cu-catalysed synthesis of substituted 3-methyleneisoindolin-1-one†
Abstract
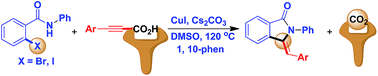
A Cu(I)-catalysed synthesis of substituted 3-methyleneisoindolin-1-ones using alkynyl acids as an alkyne source has been developed. The reaction involves the decarboxylative cross-coupling of 2-halobenzamides with aryl alkynyl acids, followed by 5-exo-dig heteroannulation. While reactions of 2-iodo benzamides proceeded without a ligand, for 2-bromo substrates, the assistance of a ligand is essential.

 Please wait while we load your content...
Please wait while we load your content...