A study on the effects of K2ZrF6 as an additive on the microstructure and hydrogen storage properties of MgH2
Abstract
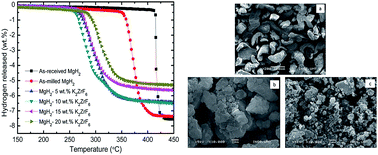
In this work, the hydrogenation properties of MgH2-doped K2ZrF6 with X wt% (X = 5, 10, 15 and 20) have been investigated for the first time. Analysis indicated that MgH2 doped with 10 wt% K2ZrF6 is the best composite for the improvement of the hydrogen storage properties of MgH2. The results showed that the onset desorption temperature after the addition of 10 wt% K2ZrF6 was 250 °C, which experienced the reduction of 100 °C and 200 °C from 350 °C and 450 °C for as-milled and as-received MgH2, respectively. The re/dehydrogenation kinetics also significantly improved compared to the un-doped MgH2. The results of the Arrhenius plot exhibited that the activation energy for the hydrogen desorption of MgH2 was reduced from 164 kJ mol−1 to 80 kJ mol−1 after the addition of 10 wt% K2ZrF6. Moreover, the X-ray diffraction spectra displayed the formation of new phases of KH and ZrH2 by the doping of K2ZrF6 with MgH2 after the dehydrogenation and rehydrogenation processes. These two compounds are believed to act as the active species and play a catalytic role in improving the hydrogen storage properties. It is, therefore, concluded that this newly developed catalytic doping system works well in improving the hydrogen sorption properties of MgH2.

 Please wait while we load your content...
Please wait while we load your content...