Effective adsorption of cationic dyes by lignin sulfonate polymer based on simple emulsion polymerization: isotherm and kinetic studies
Abstract
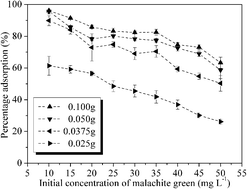
This study describes the synthesis of a lignin sulfonate polymer based on a simple emulsion polymerization from lignin sulfonates derived from the accessible by-products of paper pulp and the adsorption properties of the lignin sulfonate polymer towards dyes. Fourier transform infrared spectroscopy (FT-IR) was employed to characterize the successful synthesis of the lignin sulfonate polymer. The lignin sulfonate polymer presents selective adsorption of cationic dyes in aqueous solution, with a percentage adsorption of malachite green exceeding 95% at pH 7. The effects of pH, sorbent amount, initial malachite green concentration and temperature on the adsorption properties have been discussed. 4 kinetic models and 3 isotherm models were employed to evaluate the experimental data. The results indicated that the adsorption kinetics data fit a pseudo second-order kinetic model and the Langmuir adsorption isotherm was applicable for the adsorption of malachite green onto the lignin sulfonate polymer. Furthermore, the thermodynamic analysis (the values of ΔG0 are negative and between −20 and 0 kJ mol−1, the values of ΔH0 and ΔS0 are positive) demonstrates that the adsorption process of malachite green onto the lignin sulfonate polymer is endothermic, spontaneous and random. The results suggest that the lignin sulfonate polymer is a low-cost, alternative sorbent and has the potential to reduce the refractory chemical oxygen demand (COD) in effluents.

 Please wait while we load your content...
Please wait while we load your content...