A new approach to pyridines through the reactions of methyl ketones with 1,2,4-triazines†
Abstract
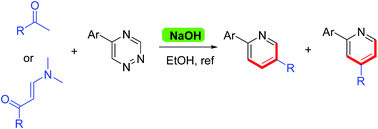
A new route to prepare pyridine derivatives based on inverse electron demand Diels–Alder/retro-Diels–Alder reactions of ketones with 1,2,4-triazines is reported. It is the first time using methyl ketones directly as a dienophile to react with 1,2,4-triazines without enamine intermediates, which is complementary to the classical Boger reaction.

 Please wait while we load your content...
Please wait while we load your content...