Azo substituted 1,2,4-oxadiazoles as insensitive energetic materials†
Abstract
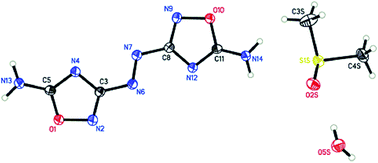
5,5′-Diamino-3,3′-azo-1,2,4-oxadiazole (3) was synthesized by reacting 3,5-diamino-1,2,4-oxadiazole with potassium permanganate in the presence of an organic solvent. Nitration of 5-amino-3-azo-1,2,4-oxadiazole gave rise to the 1,2,4-oxadiazolone (4) directly rather than the nitramine compound. Energetic salts of oxadiazolone 4 were prepared by treating with amine bases. These high nitrogen compounds were fully characterized using IR and multinuclear NMR spectroscopy, elemental analysis, and differential scanning calorimetry (DSC), and, in case of 3, with single crystal X-ray structuring. All the azo-substituted 1,2,4-oxadiazoles are impact insensitive materials.

 Please wait while we load your content...
Please wait while we load your content...