A ratiometric fluorescent probe for highly selective and sensitive detection of hypochlorite based on the oxidation of N-alkylpyridinium†
Abstract
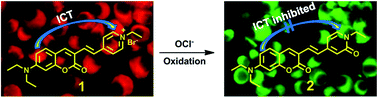
A simple and efficient ratiometric fluorescent probe 1 for hypochlorite has been rationally constructed based on a novel hypochlorite promoted oxidation of N-alkylpyridinium reaction. Notably, this oxidation reaction was first employed for developing fluorescent hypochlorite probe. Upon addition of hypochlorite, probe 1 presented a ratiometric response, with the emission wavelength displaying a remarkable blue shift (up to 143 nm). Probe 1 also provided highly selective and sensitive response to hypochlorite. The detection limit was measured to be 0.093 μM. The sensing reaction product, compound 2, was isolated and confirmed by NMR spectra and mass spectrometry. TD-DFT calculation demonstrated that the intramolecular charge transfer process in compound 2 was significantly inhibited, which result in the large blue shift of emission. Probe 1 has been successfully applied for hypochlorite detection in natural water samples. Living cell imaging experiments established that probe 1 could detect not only artificially loaded but also endogenous hypochlorite in living cells.

 Please wait while we load your content...
Please wait while we load your content...