Catalytic inactivation of alkaline phosphatase by cantharidin, an inhibitor of protein phosphatase
Abstract
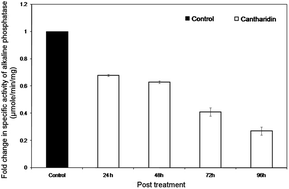
Previous investigations have shown high toxicity of cantharidin to many insects especially lepidopteran. However, its use in a higher dose for pest management has raised serious environmental concerns. Therefore, its biological potential in sub-lethal dose as a synergist was considered. It is essential for a synergist to be an effective inhibitor of metabolic enzymes, especially those responsible for biotic and abiotic stresses. As alkaline phosphatases are an important type of enzyme involved in numerous physiological processes and also in insecticide resistance, any impairment in their function may lead to serious physiological disturbances and could compromise their catalytic activity. Results showed that a sub-lethal dose of 25 μg g−1 treated artificial diet fed to Helicoverpa armigera showed inhibitory effects on catalytic activity of alkaline phosphatase in the insect midgut. Furthermore, kinetic data showed that cantharidin inhibited HaALPs competitively with respect to p-NPP. The inhibitory effect of cantharidin on the catalytic activity of ALPs as a result of its binding to the putative catalytic site was also confirmed by using homology modelling, molecular dynamics and docking simulations.

 Please wait while we load your content...
Please wait while we load your content...