A hydrazone crosslinked zwitterionic polypeptide nanogel as a platform for controlled drug delivery
Abstract
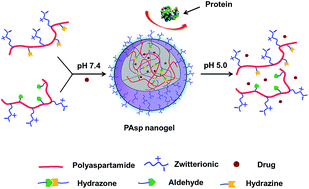
A pH-responsive polypeptide nanogel was prepared via hydrazone self-crosslinking under mild conditions. Zwitterionic sulfobetaine was introduced to the polypeptide by aminolysis reaction of polysuccinimide with N,N-dimethylaminopropylamine, and the subsequent ring-opening reaction between tertiary amine and 1,3-propanesultone. The hydrazine and aldehyde modified poly(α,β-L-aspartic acid) precursors, poly(aspartamide sulfobetaine-co-aspartylhydrazide) and poly(aspartamide sulfobetaine-co-2-oxoethyl aspartamide) were prepared by the successive aminolysis reaction of polysuccinimide. The obtained zwitterionic poly(aspartamide) nanogel exhibited excellent anti-protein adsorption ability and stability in protein solutions. An anticancer drug, doxorubicin (DOX), was entrapped into the nanogel, and showed accelerated release under acidic conditions. Additionally, the nanogel entrapped DOX could be successfully released into the cancer cells and showed high cytotoxicity. This easily prepared nanogel, with features of biocompatibility, anti-protein adsorption ability, and pH-responsiveness, is a promising controlled drug delivery system.

 Please wait while we load your content...
Please wait while we load your content...