Design, synthesis and biological evaluation of (1,3-diphenyl-1H-pyrazol-4-yl) methyl benzoate derivatives as potential BRAFV600E inhibitors
Abstract
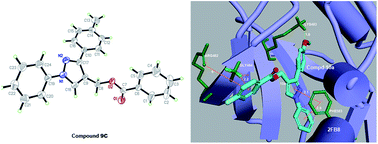
A series of (1,3-diphenyl-1H-pyrazol-4-yl) methyl benzoate derivatives (6a–10d) were designed, synthesized and evaluated as BRAFV600E inhibitors. Biological evaluation assays indicated that compound 10a showed the most potent inhibitory activity against A375, WM266.4 and BRAFV600E in vitro with IC50 values of 1.36 μM, 0.94 μM and 0.11 μM respectively compared with the positive compound vemurafenib. Furthermore, compound 10a showed highly selective BRAFV600E inhibitory activity in vitro. A docking simulation displayed that compound 10a could tightly bind the crystal structure of BRAFV600E at the active site. 3D-QSAR would provide a guideline to design and optimize more potent and positive BRAFV600E inhibitors based on the (1,3-diphenyl-1H-pyrazol-4-yl) methyl benzoate derivatives skeleton.

 Please wait while we load your content...
Please wait while we load your content...