End-group differentiating ozonolysis of furocoumarins†
Abstract
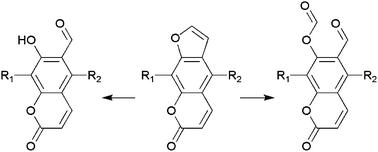
Ozonolysis of furocoumarins followed by reductive work-up yields not only common symmetrical dialdehydes, but also o-formylumbelliferones with moderate-to-high yields. Simultaneous formation of both products accounts for the transformation of carbonyl oxides – products of primary ozonide ring opening.

 Please wait while we load your content...
Please wait while we load your content...