Selective ion pair recognition of citrate and zinc ions in water by ratiometric luminescence signaling†
Abstract
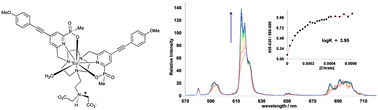
Emission from a bright europium(III) complex, based on a 1,4,7-triazacyclononane ring bearing an alkyliminodiacetate group, is modulated only when in the presence of zinc(II) ions and a carboxylate anion and was characterised by ratiometric luminescence analysis at pH 7.4. No change in emission spectral form was observed when either the metal ion or oxy-anion was added separately. Amongst the diamagnetic systems examined, the strongest binding was observed for zinc and citrate (log K = 3.9, pH 7.4) and anion selectivity was observed in the order citrate > lactate/acetate/bicarbonate. No changes in the Eu spectral characteristics were found in the presence of added Ca2+ or Mg2+ ions, consistent with the much lower affinity of these cations for the iminodiacetate binding site.

 Please wait while we load your content...
Please wait while we load your content...