Fragmentation of typical sulfonamide drugs via heterolytic bond cleavage and stepwise rearrangement†
Abstract
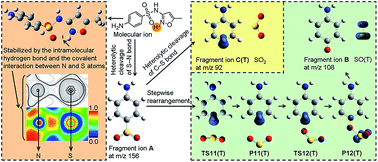
Although many experiments have been carried out to elucidate the fragmentation of typical sulfonamide drugs, little effort has been devoted to understanding the reaction process theoretically. Herein, the characteristic fragmentation pathways were investigated by performing density functional theory calculations. Employing sulfamethoxazole as a representative sulfonamide, the structure of the molecular ion was initially examined. The site of protonation was found to be located at the nitrogen atom of the isoxazole ring, which could facilitate energy minimization by forming a hydrogen bond. The fragment ion A at m/z 156 was then recognized as the result of heterolytic S–N bond cleavage with the energy requirement of 50.25 kcal mol−1. This result could be supported by analyzing the S–N bond nature and by the energy comparison of different reaction pathways. Formation of the fragment ion B at m/z 108 was attributed to a stepwise rearrangement reaction. Heterolytic C–S bond cleavage was the rate-limiting step of the overall reaction, with an energy barrier of 31.93 kcal mol−1. This is in good agreement with the Fukui function and spin density analysis. The fragment ion C(T) at m/z 92 was also obtained from the heterolytic C–S bond cleavage of A, with the energy requirement of 29.98 kcal mol−1. The results in this work suggested that formation of the fragment ions B and C(T) were competitive reactions with the major difference being the degree of the heterolytic C–S bond cleavage.

 Please wait while we load your content...
Please wait while we load your content...