Kinetics vs. thermodynamics: a unique crystal transformation from a uranyl peroxo-nanocluster to a nanoclustered uranyl polyborate†
Abstract
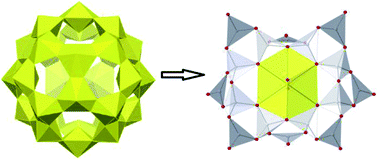
A novel method to prepare a nano-clustered uranyl polyborate in aqueous solution at room temperature has been developed. The initially formed kinetically favoured sodium uranyl peroxide yellow crystals transform, in the presence of boric acid, to the thermodynamically stable sodium uranyl polyborate in light yellow-green single crystal form.

 Please wait while we load your content...
Please wait while we load your content...