Spectrofluorimetric and TLC-densitometric methods for a stability indicating assay of valacyclovir hydrochloride in the presence of its degradation product
Abstract
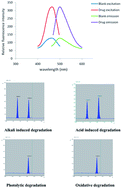
Two stability-indicating methods were developed and validated for determination of valacyclovir HCl (VAC) in the presence of its degradation product, acyclovir. The first was a TLC-densitometric method, in which chloroform : methanol : ammonia (50 : 14 : 2 v/v/v) was used as a mobile phase. Silica gel 60 F254 was used as a stationary phase and the chromatogram was scanned at 253 nm. Using this chromatographic system, VAC can be readily separated from its degradation product and gives a compact spot at an RF value of (0.55 ± 0.03). The peak area concentration plot is rectilinear over the range 20–300 ng per band. The second method represents the first attempt for spectrofluorimetric determination of VAC. The method was based on the reaction between VAC and 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) in an alkaline medium (pH 8.5) to form a highly fluorescent product that was measured at 500 nm after excitation at 465 nm. The fluorescence intensity concentration plot is rectilinear over the range 1–10 μg ml−1. The proposed methods were successfully applied for the determination of VAC in its commercial tablets with average percentage recovery of 100.13 ± 0.33 and 98.50 ± 1.75 for TLC-densitometric and spectrofluorimetric methods, respectively, without interference from common excipients. The results of the proposed methods were statistically analyzed and found to be in accordance with those given by a reported method. In addition the proposed methods were extended to a stability study of VAC, where the drug was exposed to acidic, alkaline, oxidative and photolytic degradation according to ICH guidelines.

 Please wait while we load your content...
Please wait while we load your content...