Synthesis of a recyclable and efficient Pd(ii) 4-(2-pyridyl)-1,2,3-triazole complex over a solid periodic mesoporous organosilica support by “click reactions” for the Stille coupling reaction†
Abstract
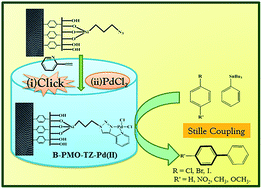
A highly efficient, reusable B-PMO-TZ-Pd(II) catalyst was synthesized by anchoring 4-(2-pyridyl)-4-(2-pyridyl)-1,2,3-triazole ligand over the surface of an organo-functionalized benzene support containing periodic mesoporous organosilica (B-PMO) via “click reaction” and the subsequent complexation with PdCl2. B-PMO materials with uniform hexagonal arrangements were prepared using C16 alkyl trimethyl ammonium bromide [CTAB] surfactant under basic conditions. The physiochemical properties of the functionalised catalyst were analysed by elemental analysis, ICP-OES, XRD, N2 sorption, TGA & DTA, solid state 13C, 29Si NMR spectra, FT-IR, XPS, UV-vis, SEM and TEM. XRD and N2 sorption revealed the morphological and textural properties of the synthesized catalyst, confirming that ordered mesoporous channel structure was retained even after the multistep synthetic procedure. The (100), (110) and (200) reflections in B-PMO are evidence of its good structural stability and the existence of long range order. The TGA-DTA results reveal that the synthesized catalyst B-PMO-TZ-Pd(II) was thermally stable, even at high temperature. The organic moieties anchored over the surface of B-PMO were demonstrated by solid state 13C NMR and FT-IR spectroscopy. Solid state 29Si NMR spectroscopy provides information about the degree of functionalization of the surface silanol group. The electronic environment and oxidation state of Pd in B-PMO-TZ-Pd(II) were monitored by XPS and UV-visible techniques. Moreover, the morphologies and topographic information of the synthesized B-PMO-TZ-Pd(II) catalyst were confirmed by SEM and TEM analyses. The catalytic properties of the catalyst for the Stille coupling reaction were screened, and higher catalytic activities with high TONs were observed. The anchored solid B-PMO-TZ-Pd(II) catalyst can be recycled efficiently and reused several (four) times without a major loss in reactivity.

 Please wait while we load your content...
Please wait while we load your content...