Synthesis, crystal structure and magnetic properties of a trinuclear phenolate bridged manganese complex containing Mn(ii)–Mn(iii) ions†
Abstract
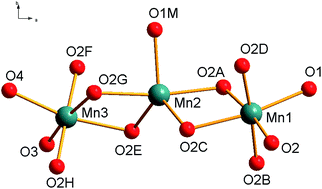
A trinuclear manganese complex containing Mn(III)–Mn(II)–Mn(III) cores, [Mn3(bp)4(CH3OH)]·0.875CH3OH·0.125H2O (1), has been prepared and structurally characterized where H2bp is an O3-donor bis(phenolate) ligand {H2bp = 2,2′-sulfinylbis(4-methylphenol)}. The combined structural and magnetic analysis reveals the mixed valence character of the compound with one central Mn(II) ion and two outer Mn(III) ions connected by phenolate bridging groups. The Mn(III)–Mn(II)–Mn(III) angle is close to a linear arrangement (∼160°) and the Mn(II)⋯Mn(III) separations are about 3.21 Å. The coordination environment around the two terminal Mn(III) ions is distorted octahedral (O6) and shows Jahn–Teller elongation, while the central Mn(II) has a distorted five coordinated trigonal-bypyramidal (O5) coordination environment. In the central Mn(II) the equatorial Mn–O bond lengths are shorter than the axial Mn–O ones. The crystal packing of 1 involves hydrogen bonding between the complex and the uncoordinated methanol molecule. The analysis of the magnetic susceptibility data is consistent with ferromagnetic intramolecular coupling among the Mn(III) and the Mn(II) ions. The system can be interpreted in terms of a simple Heisenberg–Dirac–Van Vleck model H = −J(S1S2 + S2S3) with a magnetic coupling constant of J = 0.28 cm−1.

 Please wait while we load your content...
Please wait while we load your content...