A co-precipitated Mg–Ti nano-composite with high capacity and rapid hydrogen absorption kinetics at room temperature
Abstract
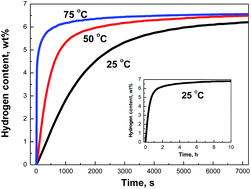
A Mg–Ti nano-composite has been co-precipitated from a tetrahydrofuran (THF) solution containing anhydrous magnesium chloride (MgCl2), titanium tetrachloride (TiCl4) and lithium naphthalide (LiNp) as the reducing agent. X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM), and pressure-composition-temperature (PCT) techniques are used to characterize phase components, microstructure and hydrogen sorption properties of the composite. The co-precipitated Mg–Ti nano-composite contains nearly 1.0 wt% of Ti distributed homogeneously on the surface or inside Mg particles having an average particle size of about 50 nm. Orthorhombic γ-MgH2 phases and tetragonal γ-TiH2 phases are obtained when the Mg–Ti nano-composite is hydrogenated at 75 °C. PCT measurements reveal the superior hydrogen absorption property of the Mg–Ti nano-composite: its maximum hydrogen capacity can reach up to 6.2 wt% within 2 h at room temperature under a hydrogen pressure of 3 MPa. The activation energy for hydrogen absorption is determined to be 50.2 kJ mol−1 H2. The hydrogenation and dehydrogenation enthalpies of the nano-composite are calculated to be −73.0 ± 1.8 and 75.8 ± 4.7 kJ mol−1 H2, close to the standard values for Mg (−74.1 ± 2.9 kJ mol−1 H2). The catalytic effects from the co-precipitated Ti and the tetragonal γ-TiH2 formed during the hydrogenation process lead to extremely fast absorption kinetics at room temperature.

 Please wait while we load your content...
Please wait while we load your content...