Facile and efficient synthesis of 1-haloalkynes via DBU-mediated reaction of terminal alkynes and N-haloimides under mild conditions†
Abstract
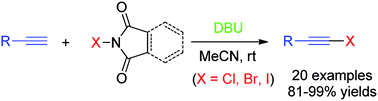
Directly from terminal alkynes and with N-halosuccinimides (halo = Br and I) or N-cholorophthalimide as the halogen sources, DBU as the activator, 1-haloalkynes were prepared in good to excellent yields at room temperature. Bis(bromoalkyne) and bis(iodoalkyne) were also synthesized in excellent yields with the NBS(NIS)/DBU combination. The reaction features inexpensive and readily available reagents, mild conditions, simple execution, extremely short reaction time, broad halogen scope, high efficiency and is metal-free. Compared to the literature reported methods, our synthetic strategy provided a greener approach towards 1-haloalkynes.

 Please wait while we load your content...
Please wait while we load your content...