Thermal analysis of aqueous urea ammonium nitrate alternative fuel
Abstract
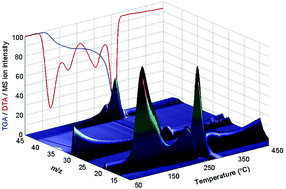
The thermal decomposition of aqueous ammonium nitrate, aqueous urea and aqueous urea ammonium nitrate was investigated by means of simultaneous TGA/DTA/DTG/MS analysis under ambient pressure, and DSC under applied pressures of 5 and 10 MPa. Aqueous urea ammonium nitrate was previously suggested as a low carbon nitrogen-based alternative fuel. Investigation of the processes which occur in the condensed phase as the temperature increases is crucial in order to understand the combustion mechanism of the suggested alternative fuel. Isomerization of urea into ammonium cyanate as well as urea hydrolysis was inhibited in the presence of ammonium nitrate, hence the fuel is considered to be chemically stable at room temperature. No solid residuals remained above 315 °C. Thermal decomposition of the fuel under ambient pressure was found to involve four principal endothermic stages: (a) water vaporization, (b) urea decomposition along with biuret formation, (c) biuret decomposition, and (d) ammonium nitrate dissociation. The thermal decomposition of the fuel under isobaric conditions of 5 and 10 MPa revealed only an exothermic process with a sharp increase in the heat flow above 300 °C. The present research increases the basic understanding of the suggested nitrogen-based alternative fuel combustion process.

 Please wait while we load your content...
Please wait while we load your content...