Effect of water on gas explosions: combined ReaxFF and ab initio MD calculations†
Abstract
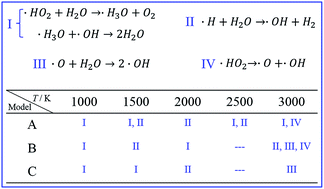
In order to understand the intrinsic effect mechanism of water addition on gas explosions, the methane explosion systems with water addition of different mole fractions were systematically studied by reactive force field and first-principles molecular dynamics (MD) simulations. The results show that the effects of water addition on a gas explosion process greatly depend on the system temperature at different reaction stages. Although the water can effectively suppress the methane oxidation process at the initial reaction stage, the same amount of water addition will obviously promote the gas explosion at the later reaction process. The ab initio MD simulations reveal that the water molecules can induce the reactions between ˙HO2 and ˙H with ˙OH radicals at the initial reaction stage. These reactions consume the reactive radicals, causing the reaction activity of the methane oxidation system to decrease. However, at a higher temperature (about 3000 K), water molecules react with ˙O and ˙H radicals to form extra ˙OH free radicals, and these ˙OH free radicals can be transferred rapidly to interact with the methane molecules by the water molecules. All these processes lead to a better reactive performance at the later reaction stage. These results not only identify the intrinsic interaction mechanism of water addition on the gas explosion system, but also provide a significant theoretical guide for the development of a highly efficient suppression method for gas explosions.

 Please wait while we load your content...
Please wait while we load your content...