Chemical loop systems for biochar liquefaction: hydrogenation of Naphthalene
Abstract
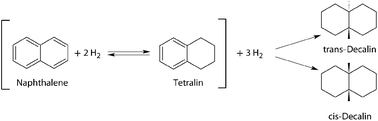
Liquefaction of biochar from liquid-phase pyrolysis was carried out in the solvent Tetralin. Tetralin is able to act as hydrogen donor during liquefaction of biochar and is itself rearranged into Naphthalene. Naphthalene must be re-hydrogenated to Tetralin to allow for further use in the liquefaction reaction (chemical loop system). Therefore Naphthalene hydrogenation was investigated, applying a full factorial design of experiments approach. The yield of Tetralin was chosen as response variable, while two-level-factors for temperature (150 °C and 200 °C), pressure (20 bar and 50 bar) and Raney-Nickel catalyst load (5 wt% and 10 wt%) were selected. The Design of Experiments approach showed a rising influence of all three factors in the order: temperature < pressure < catalyst load. The reaction kinetics of the hydrogenation of Naphthalene to Tetralin and Decalin was then investigated at 150 °C and 200 °C. The reaction proceeds stepwise and not in consecutive steps. In a first step Naphthalene reacts selectively with 96% yield to Tetralin, while the reaction of Tetralin to Decalin does not start until all Naphthalene is consumed. The rate-constant of the reaction of Naphthalene to Tetralin is one magnitude higher than that for the reaction of Naphthalene to Decalin. This is in agreement with the findings from the design of experiments approach. The results of these investigations indicate that the chemical-loop system Naphthalene–Tetralin is suitable for usage in the liquefaction of biochar.

 Please wait while we load your content...
Please wait while we load your content...