Ultrasound-assisted solventless synthesis of amines by in situ oxidation/reductive amination of benzyl halides†
Abstract
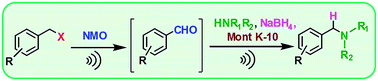
Ultrasound-assisted solventless oxidation/reductive amination of benzyl halides was developed as a facile, efficient, and environmental friendly method toward N-alkylated amines. Aldehydes were formed in situ by oxidation of organic halides with N-methylmorpholine N-oxide (NMO), followed by direct reductive amination with amines using sodium borohydride and montmorillonite K-10 catalyst as the reducing system. This green and simple procedure enables N-alkylated amines to be prepared in good to excellent yields with high selectivity of the monoalkylation.

 Please wait while we load your content...
Please wait while we load your content...