New prospects for the synthesis of N-alkyl phosphonate/phosphonic acid-bearing oligo-chitosan†
Abstract
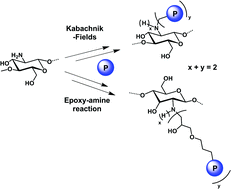
N-phosphonomethylation reactions of oligo-chitosan were performed according to Moedritzer and Kabachnik–Fields conditions. The different Moedritzer reaction conditions used did not allow the phosphonomethylation. On the contrary, the Kabachnik–Fields reactions led to oligo-chitosan methyl phosphonated derivatives. In addition, novel dialkyl phosphoryl oligo-chitosan was synthesized in water at room temperature via epoxy–amine reactions of oligo-chitosan with dialkyl (3-(oxiran-2-ylmethoxy)propyl) phosphonates. This simple and efficient synthetic method provides a new approach for the preparation of phosphonated oligo-chitosan derivatives. Then, the hydrolysis of the phosphonated compounds to generate the phosphonic acid moieties was investigated. The mildest conditions were determined in order to avoid the chitosan backbone degradation. All the products were characterized by 1H and 31P NMR analyses.

 Please wait while we load your content...
Please wait while we load your content...