Lanthanum nickel alloy catalyzed growth of nitrogen-doped carbon nanotubes by chemical vapor deposition
Abstract
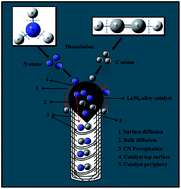
Nitrogen-doped carbon nanotubes (N-doped CNTs) were synthesized by chemical vapor deposition using lanthanum nickel (LaNi5) alloy as a catalyst and an acetylene–ammonia mixture as the carbon/nitrogen (C/N) precursor. The effects of experimental parameters such as temperature and time on the structure and yield of N-doped CNTs were studied. Transmission electron microscopy studies showed that with the increase of growth temperature and time, the C/N solubility, outer diameter and internal compartments of N-doped CNTs were increased progressively. The optimal conditions for the synthesis of N-doped CNTs were found to be 900 °C and 20 min. The elemental mapping of the catalyst tip confirmed that an intermetallic compound of lanthanum and nickel governs the growth of N-doped CNTs. X-ray photoelectron spectra revealed that the N content of CNTs varied between 3.5 and 6.9 at.% upon changing the growth temperature. Confocal Raman spectroscopy analysis showed the degree of graphitization dependence on the N doping level of CNTs. The growth of N-doped CNTs through the LaNi5 alloy catalysis was discussed on the basis of both surface and bulk diffusion mechanisms. Finally, the effect of acid treatment on the N-content and electronic structure of as-synthesized samples was investigated. These acid treated N-doped CNTs are expected to be more suitable for electrochemical applications, such as supercapacitors and the oxygen reduction reaction in fuel cells.

 Please wait while we load your content...
Please wait while we load your content...