Multicomponent diversity-oriented synthesis of symmetrical and unsymmetrical 1,4-dihydropyridines in recyclable glycine nitrate (GlyNO3) ionic liquid: a mechanistic insight using Q-TOF, ESI-MS/MS†‡
Abstract
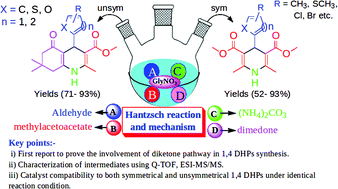
Multicomponent reactions are compelling strategies for generating a chemically diverse set of multifunctionalized heterocyclic motifs with high atom economy, rendering the transformations green. These strategies can further become more prolific if catalyst recyclability, compatibility and exploration of precise mechanistic pathways are considered. To this end, an inexpensive and recyclable glycine nitrate (GlyNO3) ionic liquid has been efficiently employed to obtain diversely substituted symmetrical and unsymmetrical 1,4-dihydropyridines with up to 93% yields via three and four components, respectively. The catalyst recyclability and compatibility to obtain both symmetrical and unsymmetrical 1,4 DHPs under identical reaction conditions are added benefits to its practical utility. Furthermore, progress of the reaction was monitored by Q-TOF, direct infusion electrospray ionization mass spectrometry (ESI-MS), and key cationic intermediates involved in the reaction have been further identified by a tandem MS experiment (Q-TOF, ESI-MS/MS), which served as the proof of concept to the mechanistic model. This is the first report which revealed that the Hantzsch reaction predominantly follows the diketone pathway among four competing reaction pathways.

 Please wait while we load your content...
Please wait while we load your content...