A family of polynuclear cobalt complexes upon employment of an indeno-quinoxaline based oxime ligand†
Abstract
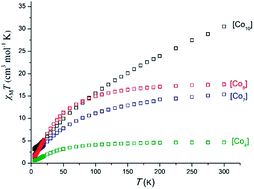
The reaction of Co(OAc)2·4H2O with LH (LH = 11H-indeno[1,2-b]quinoxalin-11-one oxime) in MeOH in the presence of NEt3 forms the complex [CoIII2CoIIO(OAc)3L3]·0.5MeOH·0.2H2O (1·0.5MeOH·0.2H2O), while repeating the reaction under solvothermal conditions yielded the heptanuclear cluster [CoII7L9 (OH)2(OAc)2.7(MeO)0.3(H2O)]·4.6MeOH·3.3H2O (2·4.6MeOH·3.3H2O). Changing the starting metal salt to Co(ClO4)2·6H2O and upon the reaction with LH in the presence of NEt3 under high temperature and pressure, we managed to isolate the decanuclear cluster [CoII10L14(OH)3.6(MeO)0.4](ClO4)2·8.5MeOH·5.75H2O (3·8.5MeOH·5.75H2O), while under normal bench conditions and upon employment of pivalates in the reaction mixture complex [CoII4L4(piv)4(MeOH)2]·MeOH·H2O (4·MeOH·H2O) was formed. Furthermore, the reaction of Co(ClO4)2·6H2O with LH and aibH (2-amino-isobutyric acid) in the presence of NEt3 in MeOH gave the mononuclear complex [CoIIIL(aib)2]·3H2O (5·3H2O), while upon increasing the metal–ligand ratio cluster [CoIII2CoIIL4(aib)2(OH)2]·7.9MeOH (6·7.9MeOH) was isolated. Finally, repeating the reaction that yielded the mononuclear complex 5·3H2O under solvothermal conditions, gave the octanuclear cluster [CoII8L10(aib)2(MeO)2](ClO4)2·6.8MeOH·7H2O (7·6.8MeOH·7H2O). Variable temperature dc magnetic susceptibility studies for complexes 2, 3, 4 and 7, reveal that all clusters display dominant antiferromagnetic interactions leading to small or diamagnetic ground-states, S.

 Please wait while we load your content...
Please wait while we load your content...