Synthesis, structure, photocatalytic and magnetic properties of an oxo-bridged copper dimer†
Abstract
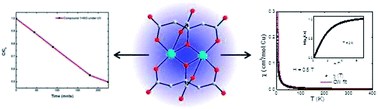
Dimeric copper complex, [GuH]4[Cu2II(Cit)2]·2H2O (where GuH = monoprotonated guanidine and Cit = citrate anion) was synthesized solvothermally and characterized by single crystal X-ray diffraction. The Cu atoms are bridged by alkoxide oxygen atoms of the citrate ligand, forming the dimer. The Cu atom adopts a distorted square pyramidal geometry. Two CuO5 units are connected edge-wise to form the dimer, which is capped by the citrate ligand. This molecular dimer is strongly H-bonded with guanidine cation and a water molecule to form a supramolecular structure. The optical band gap energy data exhibit its semiconductor behavior. We have explored this material as a photocatalyst for the degradation of organic dye. The magnetic measurements show that compound 1 behaves like a paramagnet. Detailed theoretical investigations reveal that inter-molecular H-bonding is responsible for the stability of this structure.

 Please wait while we load your content...
Please wait while we load your content...