Physical, structural, and dehydrogenation properties of ammonia borane in ionic liquids
Abstract
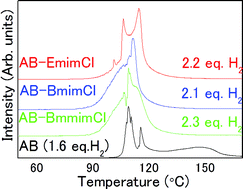
Ionic liquids (ILs) are excellent solvents for the dehydrogenation of ammonia borane (AB); however, the basic properties that allow efficient dehydrogenation are still unclear. In this report, density, viscosity, melting/freezing/glass transition temperature, solubility, and the dehydrogenation properties, including impurity gas quantification, of AB-imidazolium-based IL solutions were studied. Note that ILs can solubilize 32–35 wt% of AB, and the liquid AB–IL solutions have densities of ∼0.9 g cm−3, viscosities similar to motor oil (100–250 cP), and glass transition temperatures below −50 °C. AB–ILs are stable at room temperature for several weeks with minimal hydrogen generation, although some hydrolysis occurs immediately upon mixing as a result of trace water content. Between 80 and 130 °C, more than 2 mol H2/AB are desorbed from AB–ILs with limited impurity emissions. Furthermore, there is no reaction between AB and ILs upon dehydrogenation, and structural analysis reveals a complex solid solution.

 Please wait while we load your content...
Please wait while we load your content...