Interaction of human serum albumin with liposomes of saturated and unsaturated lipids with different phase transition temperatures: a spectroscopic investigation by membrane probe PRODAN†
Abstract
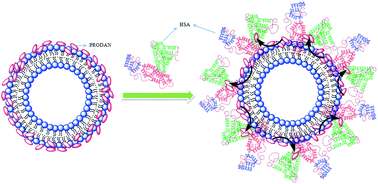
The interaction of human serum albumin (HSA) with liposomes made of saturated and unsaturated phosphocholines having distinctly different phase transition temperatures has been studied using circular dichroism (CD), steady state and time resolved fluorescence spectroscopic techniques. We used 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) as the saturated lipid and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (POPC) as the unsaturated lipid to prepare liposomes. The CD measurement reveals that the liposomes induce some kind of stabilization in HSA. The steady state and time resolved fluorescence spectra of PRODAN (6-propionyl 1,2-dimethylaminonaphthalene) were monitored to unravel the interaction between liposome and HSA. We observed that HSA partially penetrates the liposomes due to hydrophobic interaction and destabilizes the packing order of the lipid bilayer leading to leakage of the probe molecules from the liposome. It was found that HSA preferably penetrates into the liposomes, which are less prehydrated at room temperature. Thus penetration is higher in DPPC and DMPC liposomes as these liposomes are less prehydrated due to higher phase temperature (43 °C and 23 °C respectively). On the other hand HSA has less penetration in DOPC and POPC liposomes because these liposomes are more hydrated owing to lower phase transition temperature (−20 °C and −2 °C respectively). The time resolved fluorescence measurements revealed that penetration of HSA into liposomes brings about release of PRODAN molecules. Incorporation of HSA in all the liposomes results in a significant increase in the rotational relaxation time of PRODAN. This fact confirms that HSA penetrates into the liposome and forms a bigger complex.

 Please wait while we load your content...
Please wait while we load your content...