The pentafluorosulfanyl group in cannabinoid receptor ligands: synthesis and comparison with trifluoromethyl and tert-butyl analogues†
Abstract
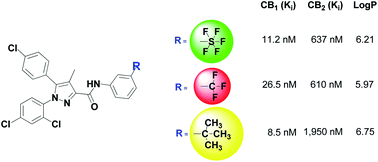
An array of cannabinoid ligands, bearing meta- and para-substituted pentafluorosulfanyl (SF5) aniline groups in position 3 of the pyrazole ring, was efficiently synthesised and compared with the exact trifluoromethyl and tert-butyl analogues. In general, the SF5 substituted ligands showed higher lipophilicity (i.e. log P values) than the CF3 counterparts and lower lipophilicity than the tert-butyl ones. In terms of pharmacological activity, SF5 pyrazoles generally showed slightly higher or equivalent CB1 receptor affinity (Ki), always in the nanomolar range, and selectivity towards the CB2 relative to both CF3 and tert-butyl analogues. Functional β-arrestin recruitment assays were used to determine equilibrium dissociation constants (Kb) and showed that all of the tested SF5 and CF3 compounds are CB1 neutral antagonists. These results confirm the possibility of successfully using an aromatic SF5 group as a stable, synthetically accessible and effective bioisosteric analogue of the electron-withdrawing CF3 group, and possibly also of bulky aliphatic groups, for drug discovery and development applications.

 Please wait while we load your content...
Please wait while we load your content...