The effect of sodium stannate as the electrolyte additive on the electrochemical performance of the Mg–8Li–1Y electrode in NaCl solution
Abstract
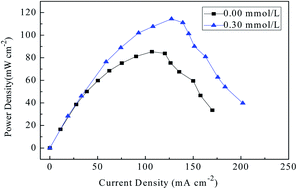
The effect of different concentrations of sodium stannate (Na2SnO3) as the electrolyte additive in 0.7 mol L−1 NaCl electrolyte solution on the electrochemical performance of the Mg–8Li–1Y alloy electrode prepared by the vacuum induction melting method have been investigated by means of potentiostatic current–time, potentiodynamic polarization, and electrochemical impedance spectroscopy measurements as well as scanning electron microscopy. The performances of the magnesium–hydrogen peroxide (Mg–H2O2) semi-fuel cells with the Mg–8Li–1Y alloy as the anode were also determined. From the study, it has been found that the corrosion potential of the Mg–8Li–1Y electrode is slightly shifted to the negative direction and the corrosion current density is markedly decreased when different concentrations of Na2SnO3 are added to a 0.7 mol L−1 NaCl electrolyte solution. The electrochemical impedance spectroscopy measurements show that the polarization resistance of the Mg–8Li–1Y electrode reduces in the following order with different concentrations of Na2SnO3: 0.20 mmol L−1 > 0.00 mmol L−1 > 0.05 mmol L−1 > 0.10 mmol L−1 > 0.30 mmol L−1. The electrochemical performance, illustrated by potentiostatic current–time curves, of Mg–8Li–1Y electrode in 0.7 mol L−1 NaCl electrolyte solution containing 0.30 mmol L−1 Na2SnO3 is better than that in 0.7 mol L−1 NaCl electrolyte solution containing Na2SnO3 in other concentrations. The addition of Na2SnO3 to the NaCl electrolyte solution loosens the product film and changes the size and thickness of the micro-clumps of the oxidation products. The Mg–H2O2 semi-fuel cell with the Mg–8Li–1Y anode in 0.7 mol L−1 NaCl solution containing 0.30 mmol L−1 Na2SnO3 presents a maximum power density of 112 mW cm−2 at room temperature.

 Please wait while we load your content...
Please wait while we load your content...