Theoretical insights into copper(i)–NHC-catalyzed C–H carboxylation of terminal alkynes with CO2: the reaction mechanisms and the roles of NHC†
Abstract
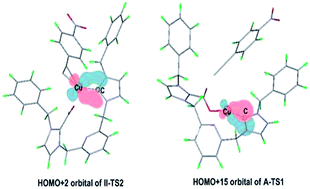
The reaction mechanisms of copper(I)–NHC-catalyzed C–H carboxylation of terminal alkynes with CO2 were investigated by DFT calculations (NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-heterocyclic carbene). Three types of reaction mechanisms were designed, explored and compared. The optimal reaction channels of corresponding pathways were selected. It was investigated that the formation of new C–C bond in the insertion process of activated CO2 by NHC was induced by the formation of Cu–O bond. Also, the functions of NHC were determined. Our calculations investigated that (1) the special difunctional roles of NHC can indeed facilitate the reaction process after the formation of CO2–NHC–Cu cocatalyst, whereas the unexpected low energy of this cocatalyst results in its ultrastability and then hinders the dropping of energy barrier in the whole reaction and (2) the additional interaction of NHC with the same metal atom will promote the insertion process of CO2 through increasing the electrophilicity of the metal center.
N-heterocyclic carbene). Three types of reaction mechanisms were designed, explored and compared. The optimal reaction channels of corresponding pathways were selected. It was investigated that the formation of new C–C bond in the insertion process of activated CO2 by NHC was induced by the formation of Cu–O bond. Also, the functions of NHC were determined. Our calculations investigated that (1) the special difunctional roles of NHC can indeed facilitate the reaction process after the formation of CO2–NHC–Cu cocatalyst, whereas the unexpected low energy of this cocatalyst results in its ultrastability and then hinders the dropping of energy barrier in the whole reaction and (2) the additional interaction of NHC with the same metal atom will promote the insertion process of CO2 through increasing the electrophilicity of the metal center.

 Please wait while we load your content...
Please wait while we load your content...