Copper catalyzed nitrile synthesis from aryl halides using formamide as a nitrile source†
Abstract
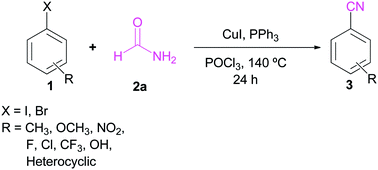
A copper iodide/triphenyl phosphine catalyzed simple and efficient protocol has been developed for cyanide free cyanation of aryl halide using formamide as a cyanide source. The reaction works well to furnish aryl nitriles using an inexpensive and easily available copper catalyst with triphenyl phosphine and phosphorus oxychloride. Under the optimized reaction conditions, a variety of electron-donating and electron-withdrawing aryl halides were efficiently converted into the respective nitriles in moderate to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...