Molecular-iodine-catalyzed aerobic oxidative synthesis of β-hydroxy sulfones from alkenes†
Abstract
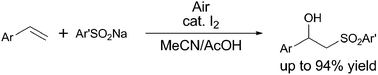
The synthesis of β-hydroxy sulfones from alkenes and sodium sulfinates under aerobic oxidative conditions was achieved in the presence of a catalytic amount of molecular iodine. Molecular oxygen in air serves as the terminal oxidant and the catalytic amount of molecular iodine acts as the sulfonyl radical initiator and peroxide reductant.

 Please wait while we load your content...
Please wait while we load your content...