Reducing polyaromatic hydrocarbons: the capability and capacity of lithium†
Abstract
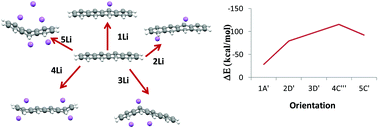
Materials which are extensively and effectively reduced by lithium atoms are of outstanding importance in the lithium battery industry and carbonaceous materials show great promise in this area. Benzene (B), naphthalene (N), anthracene (A) and tetracene (T) are considered as prototypical carbonaceous materials, which mimic sp2 allotropical forms of carbon such as graphene, graphite, carbon nanotubes (CNT) and fullerenes. We have studied lithium adsorption in these carbonaceous materials and analyzed the reduction capability of lithium by different approaches. We observed that the binding energy of these polycyclic aromatic hydrocarbon (PAH)–lithium complexes highly depends on the positioning of the lithium atoms and the size of the PAH. The sequential binding energy data show that the adsorption of the second Li atom is more facile in nature as indicated by its higher binding strengths. The NBO charge analysis also reflects that neutral lithium acquires fractional positive charges which vary from 0.4 a.u. to 2.81 a.u upon interaction with the PAH, which is a clear indication of the ability of the lithium atoms to reduce the PAH molecules. A good correlation has been obtained between the extent of charge transfer, binding and deformation energies. The current study provides the first systematic and exhaustive analysis of the sequential addition of Li atoms to carbonaceous materials and computationally estimates the reduction abilities of this class of materials.

 Please wait while we load your content...
Please wait while we load your content...