Facile synthesis and the photo-catalytic behavior of core–shell nanorods†
Abstract
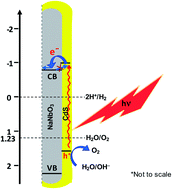
Sodium niobate nanorods (SNRs) have been synthesized by a facile surfactant free hydrothermal method. To explore their potential for photoelectrochemical water splitting under visible light, core–shell nanorods were fabricated by grafting CdS on sodium niobate nanorods. The TEM analysis shows the formation of sodium niobate nanorods which are in the order of 40 ± 5 nm in width and 1300 ± 100 nm in length. The presence of a thin layer on nanorods, as observed in a TEM image, and XRD and SAD analysis, reveals the grafting of hexagonal CdS on orthorhombic sodium niobate nanorods. This was further confirmed by dual band gap values (Eg: 3.6 for sodium niobate and 2.59 eV for CdS) determined from diffuse reflectance data of the CdS–sodium niobate nanorod sample. The CdS–sodium niobate nanorods show drastic enhancement in the current density (Jan: 7.6 mA cm−2 at 0.2 V vs. SHE) when irradiated with monochromatic UV light (300 nm), many folds higher than that observed for bare sodium niobate nanorods (Jan: 2.5 mA cm−2 at 0.2 V vs. SHE), bulk sodium niobate (Jan: 0.6 mA cm−2 at 0.2 V vs. SHE) and CdS. The conduction band (CB) minima calculations show a downhill offset of the CB edges of CdS–sodium niobate. Such a downhill staggered band gap and smooth lattice matched interface, as shown by HRTEM, seem to facilitate an efficient charge separation followed by a photo-generated e− transfer from the CdS CB to the sodium niobate CB and, therefore, appear responsible for the enhancement of the photocurrent density of CdS–sodium niobate nanorods. This is further corroborated by the time resolved photoluminescence decay measurements which show a longer average decay time (〈τ〉) for CdS–sodium niobate nanorods in the order of 8.06 ns than that for sodium niobate nanorods (6.45 ns). Furthermore, better light harvesting efficiency and incident to photon conversion efficiency (23.91% at 300 nm) observed for CdS–sodium niobate nanorods imply a better photo-generated charge carrier separation than those observed for bare sodium niobate nanorods and bulk sodium niobate. The synthesis of CdS modified sodium niobate nanorods, detailed results on the photoelectrochemical behaviour of CdS modified sodium niobate nanorods and underlying mechanism are presented.

 Please wait while we load your content...
Please wait while we load your content...