Reactions of mono- and bicyclic enol ethers with the I2–hydroperoxide system†
Abstract
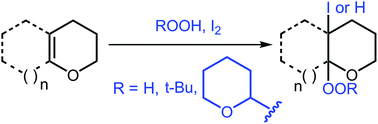
Reactions of mono- and bicyclic enol ethers with I2–H2O2, I2–ButOOH, and I2–tetrahydropyranyl hydroperoxide systems have been studied. It was shown that the reaction pathway depends on the nature of peroxide and the ring size. The reaction of 2,3-dihydrofuran and 3,4-dihydro-2H-pyran with the I2–hydroperoxide system affords iodoperoxides, α-iodolactones, and α-iodohemiacetals. Bicyclic enol ethers are transformed into vicinal iodoperoxides only in the reaction with the I2–H2O2 system, whereas the reaction with I2–ButOOH gives the hydroperoxidation product.

 Please wait while we load your content...
Please wait while we load your content...