Glycosylation with N-acetyl glycosamine donors using catalytic iron(iii) triflate: from microwave batch chemistry to a scalable continuous-flow process†‡
Abstract
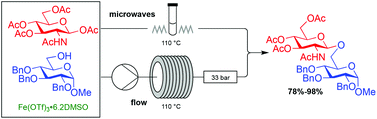
Efficient and highly selective glycosylation reactions of peracetylated β-D-N-acetyl gluco- and galactosamine are described using catalytic iron(III) triflate under microwave conditions or in a continuous flow process. Simple β-glycosides and β-(1 → 6), β-(1 → 2) and β-(1 → 3) linked disaccharides bearing various protecting groups were obtained in high yields. Insights into the glycosylation mechanism are discussed.
- This article is part of the themed collection: In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...