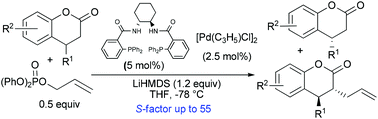
Kinetic resolution of 4-substituted-3,4-dihydrocoumarins via Pd-catalyzed asymmetric allylic alkylation reaction: enantioselective synthesis of trans-3,4-disubstituted-3,4-dihydrocoumarins†
Abstract
The kinetic resolution of 4-substituted-3,4-dihydrocoumarins was realized via Pd-catalyzed allylic substitution reaction using Trost's chiral ligand L12, affording optically active mono- and trans-3,4-disubstituted dihydrocoumarin derivatives in high yields and with high enantioselectivities with an S factor up to 55.

 Please wait while we load your content...
Please wait while we load your content...