A metal-free synthesis of diaryl-1,2-diketones by C–C triple bond cleavage of alkynones†
Abstract
A novel and environmentally benign protocol for diaryl-1,2-diketones was developed. Various diaryl-1,2-diketones were afforded in moderate to excellent yields by C–C triple bond cleavage of alkynones using molecular oxygen as an oxidant. A plausible reaction mechanism was proposed that accounts for all the experimental results. The products are important building blocks in organic synthesis and could be converted to various synthons via diverse transformations.
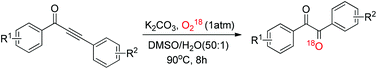

 Please wait while we load your content...
Please wait while we load your content...