Highly efficient and stereocontrolled oxidative coupling of tetrahydropyrroloindoles: synthesis of chimonanthines, (+)-WIN 64821 and (+)-WIN 64745†
Abstract
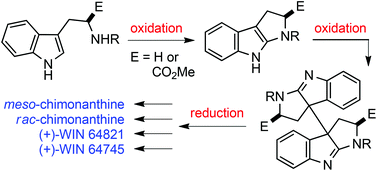
The highly efficient and stereocontrolled dimerization of 1,2,3,8-tetrahydropyrrolo[2,3-b]indoles was successfully developed with I2 as the oxidant, which allowed the rapid synthesis of meso- and rac-chimonanthines, (+)-WIN 64821 and (+)-WIN 64745 in the highest overall yields to date via the oxidation–oxidation–reduction sequence.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...