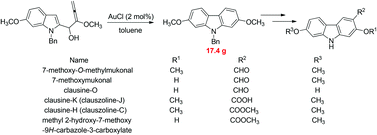
Gram scale synthesis of 7-methoxy-O-methylmukonal, clausine-O, clausine-K, clausine-H, 7-methoxymukonal, and methyl 2-hydroxy-7-methoxy-9H-carbazole-3-carboxylate†
Abstract
Naturally occurring 2,7-dioxygenated carbazole alkaloids, 7-methoxy-O-methylmukonal, clausine-O, clausine-K (clauszoline-J), clausine-H (clauszoline-C), 7-methoxymukonal and methyl 2-hydroxy-7-methoxy-9H-carbazole-3-carboxylate, have been synthesized via an efficient Au-catalyzed cyclization reaction using readily available 1-benzyl-6-methoxy-1H-indole-2-carbaldehyde and 1-methoxypropa-1,2-diene as the starting materials on the gram scale. Among them, 7-methoxymukonal and methyl 2-hydroxy-7-methoxy-9H-carbazole-3-carboxylate were prepared for the first time. In addition, the structure of methyl 2-hydroxy-7-methoxy-9H-carbazole-3-carboxylate we prepared was further confirmed by X-ray single crystal diffraction study via its ethylation reaction, which indicated that clausine-TY reported by Taufiq-Yap et al. in 2007 should be reassigned.

 Please wait while we load your content...
Please wait while we load your content...