N-Allenylnitrone acts as 2-azadiene in the Cu-catalyzed cascade reaction of O-propargylic oximes with azodicarboxylates†
Abstract
The reaction of O-propargylic oximes with azodicarboxylates efficiently afforded 1,2,4-triazine oxides in good yields. The key intermediate, N-allenylnitrone, acted as 2-azadiene, undergoing stepwise [4 + 2] cycloaddition.
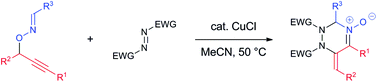

 Please wait while we load your content...
Please wait while we load your content...