1,2-Migration in the reactions of ruthenium vinyl carbene with propargyl alcohols†
Abstract
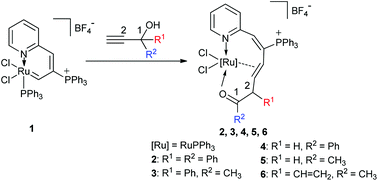
Reactions of the ruthenium vinyl carbene complex [Ru{CHC(PPh3)CH(2-Py)}Cl2PPh3]BF4 (1) with five propargyl alcohols HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(OH)R1R2, (R1 = R2 = Ph; R1 = Ph, R2 = CH3; R1 = H, R2 = Ph; R1 = H, R2 = CH3; R1 = CH
CC(OH)R1R2, (R1 = R2 = Ph; R1 = Ph, R2 = CH3; R1 = H, R2 = Ph; R1 = H, R2 = CH3; R1 = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, R2 = CH3) have been investigated, which led to the formation of several ten-membered η2-olefin coordinated ruthenacycles [Ru{O
CH2, R2 = CH3) have been investigated, which led to the formation of several ten-membered η2-olefin coordinated ruthenacycles [Ru{O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2CHR1-η2-CH
CR2CHR1-η2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(PPh3)
CHC(PPh3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(2-Py)}Cl2PPh3]BF4 (R1 = R2 = Ph, 2; R1 = Ph, R2 = CH3, 3; R1 = H, R2 = Ph, 4; R1 = H, R2 = CH3, 5; R1 = CH
CH(2-Py)}Cl2PPh3]BF4 (R1 = R2 = Ph, 2; R1 = Ph, R2 = CH3, 3; R1 = H, R2 = Ph, 4; R1 = H, R2 = CH3, 5; R1 = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, R2 = CH3, 6), respectively. In these reactions, insertion of alkynes and intramolecular 1,2-migration of propargyl alcohols were performed in tandem. The results show that the 1,2-migratory preference of the groups is in the order of H > Ph > CH3. Complexes 2, 3, 5, and 6 were characterized by X-ray diffraction analysis and NMR spectra.
CH2, R2 = CH3, 6), respectively. In these reactions, insertion of alkynes and intramolecular 1,2-migration of propargyl alcohols were performed in tandem. The results show that the 1,2-migratory preference of the groups is in the order of H > Ph > CH3. Complexes 2, 3, 5, and 6 were characterized by X-ray diffraction analysis and NMR spectra.

 Please wait while we load your content...
Please wait while we load your content...