Light-mediated, palladium-catalyzed cyclizations of unactivated 1,6-dienes†
Abstract
We report herein a light-mediated, palladium-catalyzed cyclization of unactivated 1,6-dienes under UV irradiation at rt. 1,5-Dimethylcyclopent-1-enes are formed as the only product in this transformation in moderate to excellent yields. This reaction offers a new insight into the realization of photochemical access to monocycles under ambient conditions.
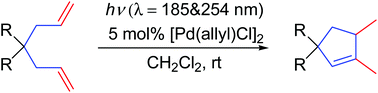

 Please wait while we load your content...
Please wait while we load your content...