Synthesis of 2-deoxy-C-glycosides via Lewis acid-mediated rearrangement of 2,3-anhydro-1-thiopyranosides†
Abstract
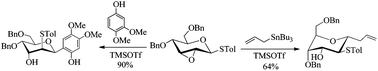
An approach to the regio- and stereo-selective construction of 2-deoxy-C-glycosides via Lewis acid-mediated rearrangement of 2,3-anhydro-1-thiopyranosides is disclosed. Treatment of 2,3-anhydro-1-thiopyranosides with phenols in the presence of TMSOTf, the migration–O-glycosylation and Fries-like O to C rearrangement took place in succession, providing aryl 2-thio-2-deoxy-C-glycosides with the single thermodynamically favourable configuration (1C4 or 4C1) in good to excellent yields. The coupling reaction of 2,3-anhydro-1-thiopyranosides with trimethylsilylated or tributylstanylated nucleophiles in the presence of TMSOTf or Sc(OTf)3, via the migration–C-glycosylation process, afforded 2-thio-2-deoxy-C-glycosides in a stereospecific manner in moderate to good yields and with the C-1 and C-2 substituents opposite. The 2-thio-functionality was further removed to produce the corresponding 2-deoxy-C-glycosides. This method may provide a convenient route for the preparation of 2-deoxy-C-glycosides.

 Please wait while we load your content...
Please wait while we load your content...